

IRP/IRE binding to the 5′ end of mRNA, which occurs in FTH, FTL, and FPN, blocks the binding of translational machinery and reduces mRNA translation. IRP/IRE binding to the 3′ end of mRNA, which occurs in TFR and DMT1, stabilizes the mRNA and enhances translation. IRPs bind to iron response elements (IRE) in the mRNA of iron regulating proteins. Cellular iron homeostasis is regulated by iron regulatory proteins (IRP). Iron is imported through the IMM to the innermost compartment, the mitochondrial matrix, where it can be utilized for heme synthesis, iron-sulfur cluster (Fe-S) biogenesis, or can be stored in mitochondrial ferritin (FtMt). Mitochondria are organelles with an outer (OMM) and inner (IMM) membrane that separate an intermembrane space (IMS). Ferrous iron may also be transported to the mitochondria. Alternatively, excess LIP iron can be stored in ferritin, a hetero-polymer of ferritin heavy (FTH) and light (FTL) chains, or can be exported from the cell by ferroportin (FPN). LIP iron can be utilized for cellular processes such as DNA synthesis, repair, and cell cycling. Divalent metal transporter 1 (DMT1) transports ferrous iron out of the endosome and into the cytoplasm were it enters a transient pool of metabolically active iron known as the labile iron pool (LIP). Within the endosome, ferric iron is converted into ferrous iron (Fe +2) by a metalloreductase Six-Transmembrane Epithelial Antigen of Prostate 3 (STEAP3). TFR and TF are recycled back to the cell surface or into the circulation, respectively. The complex is endocytosed and a reduction in pH results in dissociation of iron from the complex. Diferric-TF binds to transferrin receptors (TFR) on the plasma membrane. Differences in the permeability of the IMM and OMM result in distinct environments within these compartments.įerric iron (Fe +3) is transported through the circulation bound to a carrier protein transferrin (TF). The IMM and OMM delineate two compartments, the intermembrane space (IMS) between the IMM and OMM, and the mitochondrial matrix within the inner membrane ( Figure 1, ,2). Transfer of specific metabolites across the IMM is facilitated by hydrophobic mitochondrial carrier proteins. In contrast, the IMM forms a tight diffusion barrier to ions and metabolites and is folded into a complex form with invaginations known as cristae. The OMM is highly permeable to ions and small uncharged metabolites due to the presence of large porin channels. Similar to their bacterial ancestors, mitochondria are enclosed by a double membrane consisting of an inner (IMM) and outer membrane (OMM). During co-evolution with host cells, the mitochondrial genome has become truncated, with the result that some mitochondrial proteins are encoded by nuclear genes.
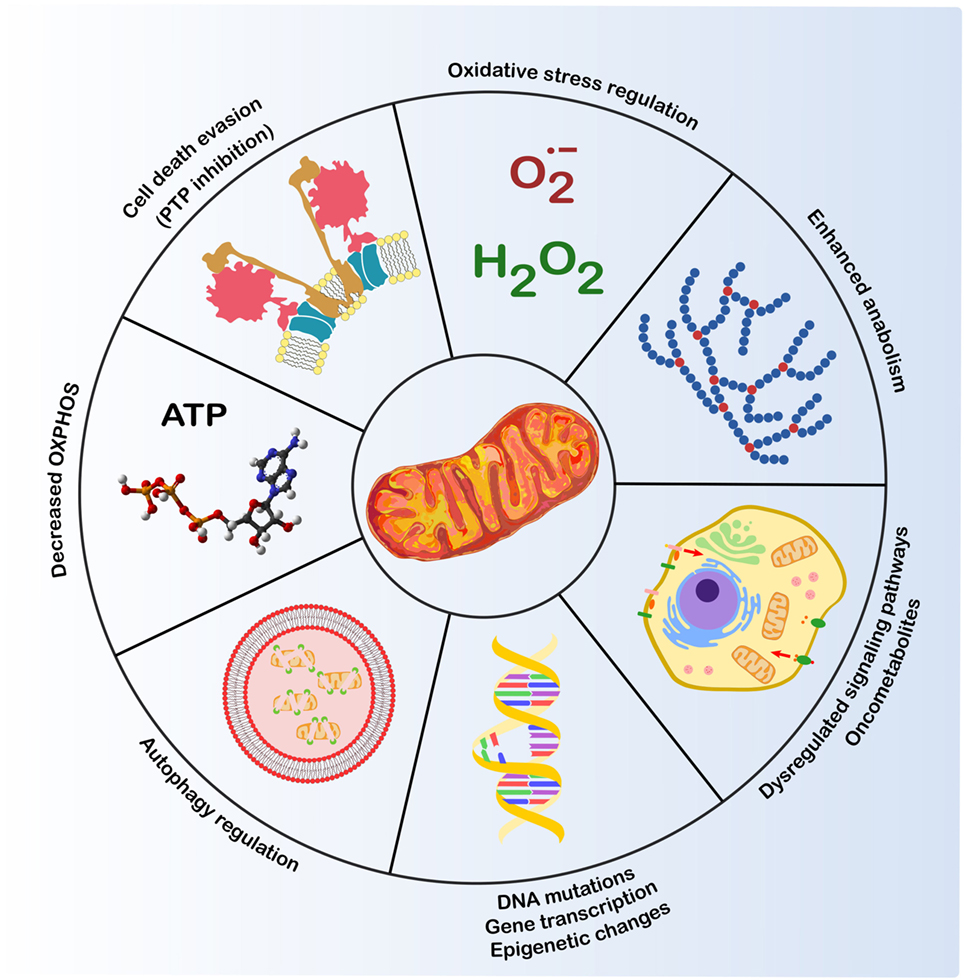
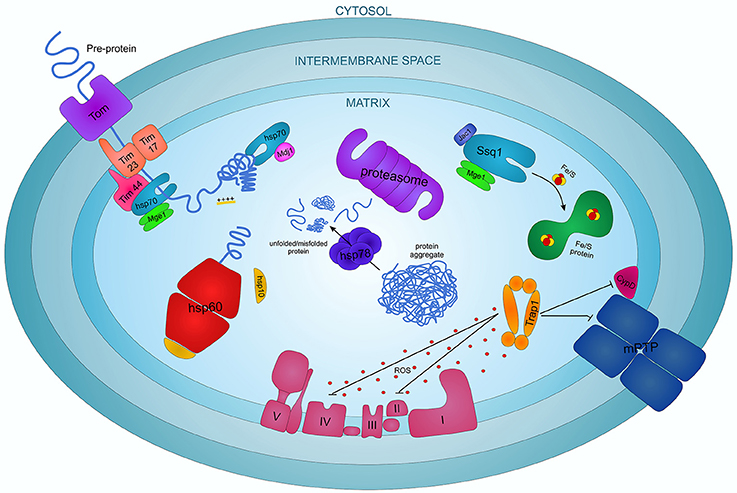
Mitochondria possess a circular DNA genome that is approximately 16 Kb. Mitochondria are ancient organelles thought to have originated from a symbiotic association between alpha-proteobacteria and a eukaryotic progenitor.


 0 kommentar(er)
0 kommentar(er)
